Regioselectivity
Addition of non-symmetrical reagent (i.e. A-B where A is not equal to B) to a non-symmetrical alkene (i.e. where the groups at each end of the double bond are different), then two isomeric products that are constitutional isomers can be obtained. For example, the reaction of HCl with propene gives 1-chloropropane and 2-chloropropane.
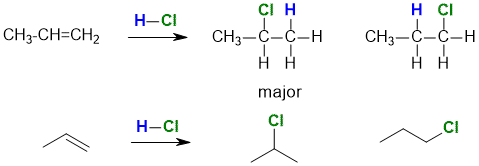
Normally, 2-chloropropane is the major product.
Since one product is favoured over the other, the reaction is said to be regioselective.
If 2-chloropropane were the only product then the reaction is said to be regiospecific.
Stereoselectivity
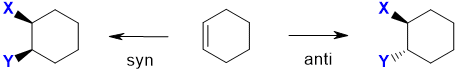
When an alkene undergoes addition, two new s bonds are formed. If we think of an alkene as having two faces, then the two new s bonds can either both form on the same face, which we call syn addition, or they can be formed on different faces which we call anti addition. Remember that an alkene unit is planar. The alkene has been drawn in a wedge hash type style which means that the substituents on the C=C are into and out of the page. This means that the two faces of the alkene are above and below the where the alkene makes the horizontal plane. Syn addition results when the two new bonds are both formed to the same face of the alkene, in this case they are shown to have formed on the top face. Anti addition results when the two new bonds have formed to opposite faces.
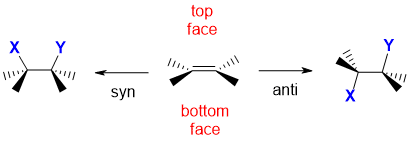
Note : This is a simplification.... in the case of a simple alkene like ethene, the free rotation of the C-C bond means that the two product structures are conformational isomers and rapidly inconvert. However with cyclic alkenes, where the ring structure prevents the free rotation, the two products are stereoisomers and are more obvious.
Addition of non-symmetrical reagent (i.e. A-B where A is not equal to B) to a non-symmetrical alkene (i.e. where the groups at each end of the double bond are different), then two isomeric products that are constitutional isomers can be obtained. For example, the reaction of HCl with propene gives 1-chloropropane and 2-chloropropane.

Since one product is favoured over the other, the reaction is said to be regioselective.
If 2-chloropropane were the only product then the reaction is said to be regiospecific.
Stereoselectivity
When an alkene undergoes addition, two new s bonds are formed. If we think of an alkene as having two faces, then the two new s bonds can either both form on the same face, which we call syn addition, or they can be formed on different faces which we call anti addition. Remember that an alkene unit is planar. The alkene has been drawn in a wedge hash type style which means that the substituents on the C=C are into and out of the page. This means that the two faces of the alkene are above and below the where the alkene makes the horizontal plane. Syn addition results when the two new bonds are both formed to the same face of the alkene, in this case they are shown to have formed on the top face. Anti addition results when the two new bonds have formed to opposite faces.



you are clear my mind actually after reading your article i got clear my complete doubt. thanks for such easy understanding post. Sharing on What is the difference between structural and conformational isomers? for future aspect at here http:// electrotopic. com/what-is-the-difference-between-structural-and-conformational-isomers/
ReplyDelete