
Sunday, October 6, 2019
Flavonoids

Friday, October 4, 2019
Bacteriochlorin
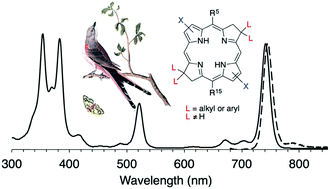
Bacteriochlorins such as bacteriochlorophyll a absorb strongly in the near-infrared spectral region and are potentially useful in a variety of photochemical fields.
De novo syntheses of bacteriochlorins entail self-condensation of a dihydrodipyrrin-acetal (containing one pyrrole and one pyrroline joined via a methylidene bridge) either via a heavily studied Eastern–Western (E–W) route or a recently reported Northern–Southern (N–S) route.
The Michael addition to form the dihydrodipyrrin-acetal for the E–W approach has limited scope for the installation of substituents on the pyrroline units. By use of the N–S route, new bacteriochlorins have been prepared that bear a pair of aryl or alkyl groups, together termed a “swallowtail” substituent, at each β-pyrroline unit, a previously inaccessible design.
Single-crystal X-ray structures of three intermediates were determined. Bacteriochlorins synthesized herein exhibit characteristic bacteriochlorophyll-like absorption spectra, including a Qy band in the region of 730–758 nm.
The swallowtail groups have little impact on the excited-state properties of the bacteriochlorins, and the slight changes of spectral properties that are observed stem from substituent electronic effects rather than changes in structure.
In summary, introduction of an integral swallowtail unit on the pyrroline ring opens new sites for tailoring molecular designs without altering the attractive photophysical features of the synthetic bacteriochlorins.
Chlorins are porphyrins with one beta-saturated pyrrolic ring
destroying the p-conjugation at the beta-carbons. The saturation
of the beta-carbons e†ectively prevents the ring current from
taking the outer route at that particular pyrrolic unit. Since
chlorins have 24p-electrons, all p-electrons cannot directly
participate in the aromatic pathway. However, when one considers the aromatic pathway to be a superposition of several
22p-electron paths, all 24p-electrons can form the total aromatic pathway. For chlorins, pyrrolic rings with an inner
hydrogen have strong local ring currents. For and t-CH2
(a) cCH the pyrrolic ring without an inner hydrogen has a 2
(b),
NICS values which is twice as large as for the corresponding
unit in indicating a stronger local ring current (see t-PH2
,
Table 4). This can be explained by assuming that the aromatic
pathway consists of a superposition of two 22p-electron pathways. In for example, the two 22 t-CH p-electron routes 2
(a),
consist of six p-electrons from one aromatic pyrrolic ring with
an inner hydrogen, Ðve p-electrons from the pyrrolic ring
without an inner hydrogen, four p-electrons from the meso
carbons, four p-electrons from the outer path of the other pyrrolic ring with an inner hydrogen, and three p-electrons from
the inner path of the b-saturated pyrrolic ring. Another 22pelectron path consists of the two pyrrolic rings with an inner
hydrogen, and the inner paths at the two other pyrrolic rings.
For the NICS value for the pyrrolic ring without an c-CH2
,
inner hydrogen is only 2.8 ppm, indicating that the inner
paths at the pyrrolic rings without an inner hydrogen is the
dominating one. For the chlorins with an inner hydrogen connected to the b-saturated ring, the ring current is signiÐcantly
weaker than for the other chlorins. For and t-CH2
(a) c-CH2
(a),
the obtained current susceptibilities are 7.4 and 7.2 nA T~1,
respectively, while for the two other chlorins the corresponding values are 4.6 and 4.9 nA T~1.
Bacteriochlorins have 22p-electrons and consist of four pyrrolic rings of which two are b-saturated. The two transFig. 8 (a) The 18p-[16]annulene internal cross pathway, (b) the traditional 18p-[18]annulene pathway, (c) the most important 22pelectron pathway and (d) the 26p-electron aromatic pathway for t-PH2bacteriochlorins have large current susceptibilities, while the current susceptibility for and is 5.3 and 3.1 c-BCH2 t-IBCH2 nA T~1, respectively. The aromatic pathway for con- t-BCH2 (a) sists of two aromatic pyrrolic rings with an inner hydrogen and the inner path at the b-saturated rings yielding a 22pelectron pathway. For the pyrrolic rings without an t-BCH2 (b), inner hydrogen have large local ring currents. The aromatic pathway for consists of the two aromatic pyrrolic t-BCH2 (b) rings without an inner hydrogen and the inner paths at the b-saturated rings. Since for the current must pass t-BCH2 (b), two inner hydrogens the ring-current susceptibility is 7.8 nA T~1 as compared to 9.2 nA T~1 for has t-BCH2 (a). t-BCH2 (a) current susceptibility almost as large as It is question- t-PH2 . able whether with a current susceptibility of 3.1 nA t-IBCH2 T~1 can be considered aromatic. D. t-3BCH and 2 , t-4BCH2 t-PH2 (m) The molecules have three t-3BCH b-saturated pyrrolic rings 2 yielding 20 p-electrons. The only possible current pathway consisting of (4n ] 2) p-electrons is the 18p-[16]annulene inner cross path. The current susceptibilities for the studied t-3BCH molecules are only 2.5 and 3.3 nA T~1, respectively, 2 which indicates that they are not particularly aromatic. Energetically, lies lower than since the t-3BCH2 (a) t-3BCH2 (b), unsaturated pyrrolic ring with an inner hydrogen is much more aromatic than the pyrrolic ring without the inner hydrogen. Hence, is more stable than which t-3BCH2 (a) t-3BCH2 (b), does not have any inner hydrogen connected to the bsaturated ring. The molecules do not have a signiÐ- t-3BCH2 cant 18p-[16]annulene inner cross aromatic pathway. The current susceptibility of of 7.2 nA T t-4BCH ~1 is of 2 the same size as for and For the t-CH2 (a) c-CH2 (a). t-4BCH2 , only possible aromatic pathway is the 18p-annulene inner cross path. As expected, has a smaller ring-current t-4BCH2 radius than the other porphyrins studied. is also the t-4BCH2 only molecule among the studied ones which has a signiÐcant 18p-[16]annulene inner cross aromatic pathway. The reason is that the porphyrins in general are stabilized by the local aromaticity of the pyrrolic rings, and their aromaticity would be weakened by the 18p-[16]annulene pathway. In t-4BCH2 , all pyrrolic rings are non-aromatic and the 18p-[16]annulene aromatic pathway stabilizes the molecule energetically. In Table 2, it can be seen that is the most destabi- t-4BCH2 lized molecule as compared to the other porphyrins. Even though has a relatively strong ring current, it lies t-4BCH2 energetically high. The hydrogenation energy is 79.3 kJ mol~1 larger when two hydrogens are added to than when t-3BCH2 (a) two hydrogens are added to yielding The t-PH2 t-CH2 (a). general trend for the hydrogenation of the b-carbons for the porphyrins is that the energy gain decreases with the number of b-saturated rings. The molecule has a very large current radius, since t-PH2 (m) the two pyrrolic rings with an inner hydrogen simulate a global ring current. The saturated meso carbons prevent the global ring current, which is also reÑected on the ARCS value. From the NICS values in Table 4 one can see that the rings without an inner hydrogen are not particularly aromatic, which indicates that the pyrrolic rings without an inner hydrogen need the conjugated p-electron environment in order to sustain a local ring current. The obtained current susceptibility for is 3.0 nA T t-PH ~1, which shows that the 2 (m) contribution from the pyrrolic rings to the ARCS values of the porphyrins is in general much smaller than the contribution from the porphyrin loop. VI. Summary The present study shows that the total aromatic pathway of porphyrins must be considered as a superposition of several pathways. For porphin, all 26p-electrons are part of the aromatic pathway. The pathway must be considered mainly as a superposition of the 26p-electron path and two 22p-electron paths. For chlorins, all 24p-electrons participate in the aromatic pathway, which implies that the total aromatic pathway must be considered to consist of a superposition of 22pelectron paths. For bacteriochlorins, all 22p-electrons are involved in the aromatic pathway. The b-unsaturated pyrrolic rings in porphyrins have local ring currents which are intergraded parts of the total aromatic pathway. The current strength of the pyrrolic rings with an inner hydrogen is of about the same size as for the free pyrrole molecule. Pyrrole rings without an inner hydrogen have signiÐcantly smaller local ring currents than the rings with an inner hydrogen. The porphyrins are energetically stabilized by the aromaticity of the pyrrolic rings. The 18p-[16]annulene inner cross aromatic pathway does not exist in porphyrins until all pyrrolic rings are nonaromatic. This means that the units of the pyrrolic rings C2 H2 do not function as exocyclic bridges. We also found that the 1H NMR shieldings of the inner hydrogens correlate well with the calculated current susceptibilities, and thus can be used as an experimental measure for the aromaticity of free-base porphyrins.
Bacteriochlorins have 22p-electrons and consist of four pyrrolic rings of which two are b-saturated. The two transFig. 8 (a) The 18p-[16]annulene internal cross pathway, (b) the traditional 18p-[18]annulene pathway, (c) the most important 22pelectron pathway and (d) the 26p-electron aromatic pathway for t-PH2bacteriochlorins have large current susceptibilities, while the current susceptibility for and is 5.3 and 3.1 c-BCH2 t-IBCH2 nA T~1, respectively. The aromatic pathway for con- t-BCH2 (a) sists of two aromatic pyrrolic rings with an inner hydrogen and the inner path at the b-saturated rings yielding a 22pelectron pathway. For the pyrrolic rings without an t-BCH2 (b), inner hydrogen have large local ring currents. The aromatic pathway for consists of the two aromatic pyrrolic t-BCH2 (b) rings without an inner hydrogen and the inner paths at the b-saturated rings. Since for the current must pass t-BCH2 (b), two inner hydrogens the ring-current susceptibility is 7.8 nA T~1 as compared to 9.2 nA T~1 for has t-BCH2 (a). t-BCH2 (a) current susceptibility almost as large as It is question- t-PH2 . able whether with a current susceptibility of 3.1 nA t-IBCH2 T~1 can be considered aromatic. D. t-3BCH and 2 , t-4BCH2 t-PH2 (m) The molecules have three t-3BCH b-saturated pyrrolic rings 2 yielding 20 p-electrons. The only possible current pathway consisting of (4n ] 2) p-electrons is the 18p-[16]annulene inner cross path. The current susceptibilities for the studied t-3BCH molecules are only 2.5 and 3.3 nA T~1, respectively, 2 which indicates that they are not particularly aromatic. Energetically, lies lower than since the t-3BCH2 (a) t-3BCH2 (b), unsaturated pyrrolic ring with an inner hydrogen is much more aromatic than the pyrrolic ring without the inner hydrogen. Hence, is more stable than which t-3BCH2 (a) t-3BCH2 (b), does not have any inner hydrogen connected to the bsaturated ring. The molecules do not have a signiÐ- t-3BCH2 cant 18p-[16]annulene inner cross aromatic pathway. The current susceptibility of of 7.2 nA T t-4BCH ~1 is of 2 the same size as for and For the t-CH2 (a) c-CH2 (a). t-4BCH2 , only possible aromatic pathway is the 18p-annulene inner cross path. As expected, has a smaller ring-current t-4BCH2 radius than the other porphyrins studied. is also the t-4BCH2 only molecule among the studied ones which has a signiÐcant 18p-[16]annulene inner cross aromatic pathway. The reason is that the porphyrins in general are stabilized by the local aromaticity of the pyrrolic rings, and their aromaticity would be weakened by the 18p-[16]annulene pathway. In t-4BCH2 , all pyrrolic rings are non-aromatic and the 18p-[16]annulene aromatic pathway stabilizes the molecule energetically. In Table 2, it can be seen that is the most destabi- t-4BCH2 lized molecule as compared to the other porphyrins. Even though has a relatively strong ring current, it lies t-4BCH2 energetically high. The hydrogenation energy is 79.3 kJ mol~1 larger when two hydrogens are added to than when t-3BCH2 (a) two hydrogens are added to yielding The t-PH2 t-CH2 (a). general trend for the hydrogenation of the b-carbons for the porphyrins is that the energy gain decreases with the number of b-saturated rings. The molecule has a very large current radius, since t-PH2 (m) the two pyrrolic rings with an inner hydrogen simulate a global ring current. The saturated meso carbons prevent the global ring current, which is also reÑected on the ARCS value. From the NICS values in Table 4 one can see that the rings without an inner hydrogen are not particularly aromatic, which indicates that the pyrrolic rings without an inner hydrogen need the conjugated p-electron environment in order to sustain a local ring current. The obtained current susceptibility for is 3.0 nA T t-PH ~1, which shows that the 2 (m) contribution from the pyrrolic rings to the ARCS values of the porphyrins is in general much smaller than the contribution from the porphyrin loop. VI. Summary The present study shows that the total aromatic pathway of porphyrins must be considered as a superposition of several pathways. For porphin, all 26p-electrons are part of the aromatic pathway. The pathway must be considered mainly as a superposition of the 26p-electron path and two 22p-electron paths. For chlorins, all 24p-electrons participate in the aromatic pathway, which implies that the total aromatic pathway must be considered to consist of a superposition of 22pelectron paths. For bacteriochlorins, all 22p-electrons are involved in the aromatic pathway. The b-unsaturated pyrrolic rings in porphyrins have local ring currents which are intergraded parts of the total aromatic pathway. The current strength of the pyrrolic rings with an inner hydrogen is of about the same size as for the free pyrrole molecule. Pyrrole rings without an inner hydrogen have signiÐcantly smaller local ring currents than the rings with an inner hydrogen. The porphyrins are energetically stabilized by the aromaticity of the pyrrolic rings. The 18p-[16]annulene inner cross aromatic pathway does not exist in porphyrins until all pyrrolic rings are nonaromatic. This means that the units of the pyrrolic rings C2 H2 do not function as exocyclic bridges. We also found that the 1H NMR shieldings of the inner hydrogens correlate well with the calculated current susceptibilities, and thus can be used as an experimental measure for the aromaticity of free-base porphyrins.
Chlorin

A chlorin is a tetrapyrrole. Chlorins are partially hydrogenated versions of porphyrins.] The parent chlorin is a rare compound, but substituted chlorins are common. Magnesium-containing chlorins are called chlorophylls. Chlorophylls are the photosensitive pigment in chloroplasts.
Related to chlorins are bacteriochlorins and isobacteriochlorins. They are found as the core of some bacteriochlorophylls. These tetrapyrroles are further reduced (hydrogenated) relative to chlorins.
Because of their photosensitivity, chlorins are in active use as photosensitizing agents in experimental photodynamic therapy
Subscribe to:
Posts (Atom)

