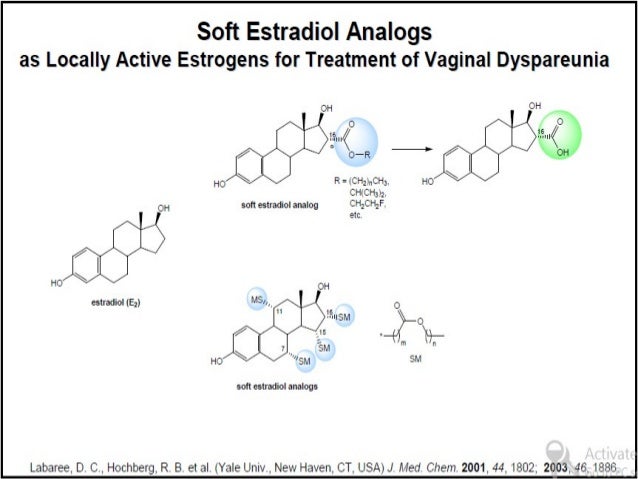
Pharmacol Rep. 2006 Sep-Oct;58(5):599-613.
Monday, November 20, 2017
Pro Drug
A prodrug is a pharmacological substance (drug) that is administered in an inactive (or significantly less active) form. Once administered, the prodrug is metabolised in vivo into an active metabolite. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and excretion (ADME) optimization. Prodrugs are usually designed to improve oral bioavailability, with poor absorption from the gastrointestinal tract usually being the limiting factor.
Additionally, the use of a prodrug strategy increases the selectivity of the drug for its intended target. An example of this can be seen in many chemotherapytreatments, in which the reduction of adverse effects is always of paramount importance. Drugs used to target hypoxic cancer cells, through the use of redox-activation, utilise the large quantities of reductase enzyme present in the hypoxic cell to convert the drug into its cytotoxic form, essentially activating it. As the prodrug has low cytotoxicity prior to this activation, there is a markedly lower chance of it "attacking" healthy, non-cancerous cells which reduces the side-effects associated with these chemotherapeutic agents.
In rational drug design, the knowledge of chemical properties likely to improve absorption and the major metabolic pathways in the body allows the modification of the structure of new chemical entities for improved bioavailability. Sometimes the use of a prodrug is unintentional, however, especially in the case of serendipitousdrug discoveries, and the drug is only identified as a prodrug after extensive drug metabolism studies.
Prodrugs and Soft Drugs
Prodrugs
Prodrugs are pharmacologically inactive derivatives of active drugs. They are designed to maximize the amount of active drug that reaches its site of action, through manipulation of the physicochemical, biopharmaceutical or pharmacokinetic properties of the drug. Prodrugs are converted into the active drug within the body through enzymatic or non-enzymatic reactions.
Soft drugs
Drugs are sometimes divided into "hard drugs " and "soft drugs". Hard drugs are "non-metabolizable drugs" or drugs which are metabolized to biologically active metabolites. The metabolites of hard drugs are frequently toxic oxidation products. Soft drugs are drugs which are characterized by a predictable and controllable in vivo destruction (i.e. metabolism) to non-toxic products after they have achieved their therapeutic role.
Similarly "hard compounds" can be defined as compounds which do not degrade in the environment or compounds which do it very slowly. Thus, these compounds will lead to progressive pollution of the environment. An example of a hard compound is the insecticide DDT.
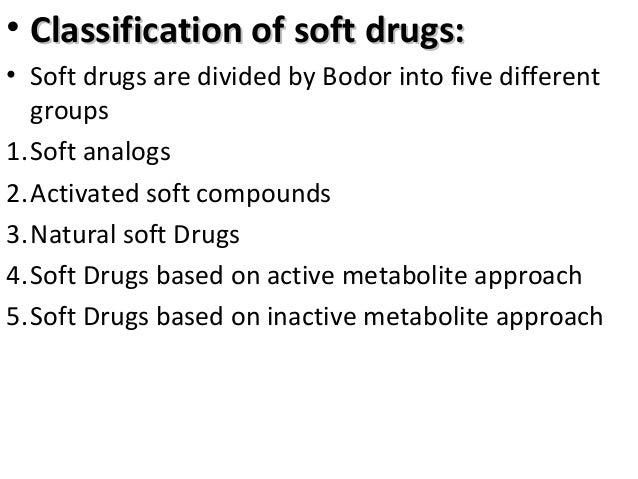
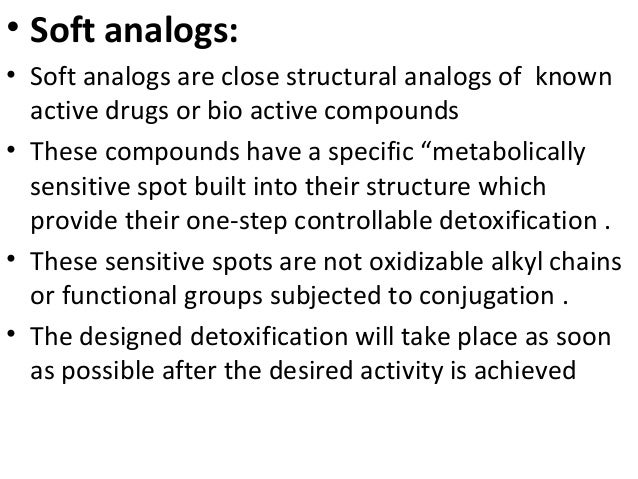
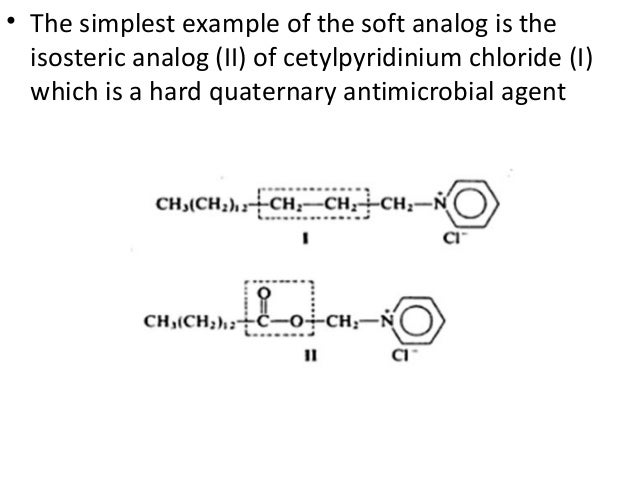
"Soft compounds" can be defined as biologically active compounds which are readily degraded to non-toxic and biologically inactive degradation products in the environment. The purpose of this project is to design, synthesise and test soft drugs and soft environmental-friendly compounds
How are drugs designed and developed
What is a drug?
- Drugs are chemical or biological substances that have some kind of physiological? or biochemical? effect on our bodies.
- They may be single compounds or a mixture of different compounds.
- Their effects are intended to be beneficial but can cause harmful side effects in some people.
- All drugs interact with specific ‘targets’ in the body, with the aim of modifying their activity and often resulting in a therapeutic?effect. For example, pain relief.
- Drug targets are usually proteins? but are in some cases small regions of DNA? or RNA?.
- Drugs work either by stimulating or blocking the activity of their targets.
How is a drug developed?
- The development of a new therapeutic drug is a complex, lengthy and expensive process.
- It can take 10-15 years and over £500 million to develop a drug from an initial concept, test its safety and effectiveness in humans and then get it into the hospital market, this includes:
- 2-4 years of pre-clinical development
- 3-6 years of clinical development
- additional time for dealing with the regulatory authorities.
Stage 1: Drug discovery
- The first stage of the drug development process is drug discovery.
- In the past, some drugs have been discovered by accident, for example, penicillin.
- Today, more systematic approaches are used, such as:
- high-throughput screening: which allows scientists to test thousands of potential targets with thousands of diverse chemical compounds to identify a new drug-target combination.
- rational drug design: which involves designing and synthesising compounds based on the known structure of a specific target molecule.
- While high-throughput screening may identify hundreds of potential lead components, many will be eliminated at the first round of testing. During this round compounds are tested in cultured cells or animals to find out how effective they are and whether they have any toxic effects.
- Rational drug design develops fewer compounds compared to high-throughput screening. However, these compounds are very specific to the target and use computer-based modelling to achieve this specificity.
Stage 2: Pre-clinical development
- Pre-clinical testing is used to determine how best to develop the drug for its intended use.
- It aims to establish how drugs are absorbed and distributed in the body, and how they are broken down and removed from the body.
- If appropriate, promising drugs may be modified in an attempt to improve their properties in subtle ways in a process called lead optimisation.
- The results of pre-clinical testing are also used to determine how to best formulate the drug for its intended clinical use, for example whether it would be most effective as a cream, a pill, an injection or a spray.
- The pre-clinical studies aim to whittle hundreds of compounds down to just a few useful candidate drugs.
- These few drugs will then be submitted to the appropriate regulatory authorities and, if accepted, the compound can be taken on to clinical development.
Stage 3: Clinical development
- This is divided into Phases 0, I, II, III and IV.
- Clinical development, also known as clinical trials, involves testing the drug on human volunteers to provide more information about its safety and effectiveness.
- By the end of the clinical development phase, most of the investigational new drugs will have been eliminated on the grounds of safety and effectiveness.
- Only one or two compounds will be submitted as a new drug application. In the UK, this is known as a market authorisation application.
- After a drug has been approved by the appropriate regulatory bodies, pharmaceutical companies have a short period where only they have the rights to market the drug (exclusivity) and before other companies can market the same drug.
- This exclusivity period is used to regain the massive investment required to develop and launch the new drug.
- After full approval, drug companies must continue to test their drug and monitor feedback from healthcare professionals to ensure the safety and effectiveness of the drug.
- After launching a drug, new side effects or risk factors may be identified that had not been previously recorded. This is Phase IV of clinical development and is part of the continued monitoring of the effectiveness of the drug in their target patients.

An illustration showing the different stages involved in developing a drug.
Sunday, November 12, 2017
Regioselectivity and Stereoselectivity
Regioselectivity
Addition of non-symmetrical reagent (i.e. A-B where A is not equal to B) to a non-symmetrical alkene (i.e. where the groups at each end of the double bond are different), then two isomeric products that are constitutional isomers can be obtained. For example, the reaction of HCl with propene gives 1-chloropropane and 2-chloropropane.
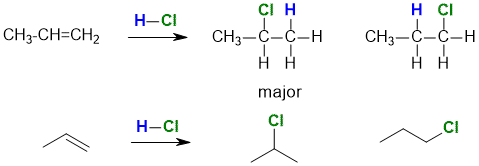
Normally, 2-chloropropane is the major product.
Since one product is favoured over the other, the reaction is said to be regioselective.
If 2-chloropropane were the only product then the reaction is said to be regiospecific.
Stereoselectivity
When an alkene undergoes addition, two new s bonds are formed. If we think of an alkene as having two faces, then the two new s bonds can either both form on the same face, which we call syn addition, or they can be formed on different faces which we call anti addition. Remember that an alkene unit is planar. The alkene has been drawn in a wedge hash type style which means that the substituents on the C=C are into and out of the page. This means that the two faces of the alkene are above and below the where the alkene makes the horizontal plane. Syn addition results when the two new bonds are both formed to the same face of the alkene, in this case they are shown to have formed on the top face. Anti addition results when the two new bonds have formed to opposite faces.
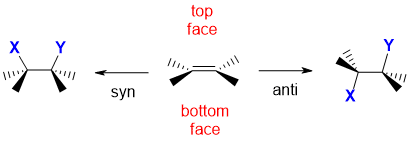
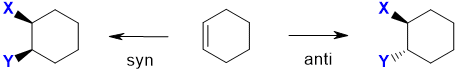
Note : This is a simplification.... in the case of a simple alkene like ethene, the free rotation of the C-C bond means that the two product structures are conformational isomers and rapidly inconvert. However with cyclic alkenes, where the ring structure prevents the free rotation, the two products are stereoisomers and are more obvious.
Addition of non-symmetrical reagent (i.e. A-B where A is not equal to B) to a non-symmetrical alkene (i.e. where the groups at each end of the double bond are different), then two isomeric products that are constitutional isomers can be obtained. For example, the reaction of HCl with propene gives 1-chloropropane and 2-chloropropane.

Since one product is favoured over the other, the reaction is said to be regioselective.
If 2-chloropropane were the only product then the reaction is said to be regiospecific.
Stereoselectivity
When an alkene undergoes addition, two new s bonds are formed. If we think of an alkene as having two faces, then the two new s bonds can either both form on the same face, which we call syn addition, or they can be formed on different faces which we call anti addition. Remember that an alkene unit is planar. The alkene has been drawn in a wedge hash type style which means that the substituents on the C=C are into and out of the page. This means that the two faces of the alkene are above and below the where the alkene makes the horizontal plane. Syn addition results when the two new bonds are both formed to the same face of the alkene, in this case they are shown to have formed on the top face. Anti addition results when the two new bonds have formed to opposite faces.


Anchimeric Assistance (Neighboring Group Participation)
Anchimeric Assistance (Neighboring Group Participation)
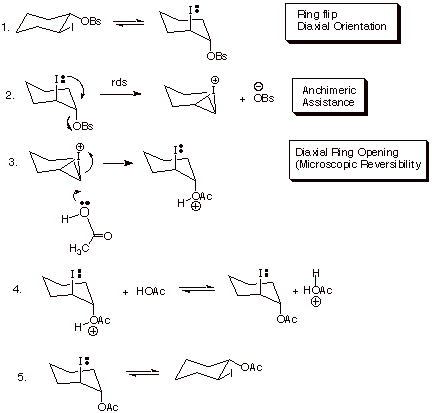
The participation of neighboring groups in an SN reaction is revealed by unique stereochemical results (retention in the substitution process) and also (usually) by obvious rate enhancements in comparison to a model in which neighboring group participation would be stereoelectronically impossible. Consider the case of trans-2-iodocyclohexyl brosylate (remember, brosylate is p-bromobenzenesulfonate, a very good leaving group, better even than tosylate (why?)).
Classical and Non Classical Carbacation
Here is a picture of a "classical" carbocation, there is an electron deficient carbon bearing a positive charge.

There are many examples of "non-classical" carbocations, but the 2-norbornyl carbocation is among the best known.
Labeling experiments have shown that the positive charge resides on more than one carbon in the 2-norbornyl ion. Early on, the data was explained by equilibrating classical ions, but soon another possibility emerged - one involving a single non-classical ion.
The problem comes down to: are the equilibrating classical ions ground state structures with the non-classical ion serving as the transition state, or is the non-classical ion the ground state? This debate went on for a very long period of time, but now most agree that the non-classical structure is the ground state in the 2-norbornyl system. In fact, a recent, and difficult to obtain, crystal structure for the 2-norbornyl cation has been published proving that the ion exists with the non-classical geometry (thanks to Klaus for finding this reference, see his comment below).

A key difference between classical and non-classical structures is the bonding. As illustrated above, a classical ion has a carbon with a sextet of electrons and 3 other bonds. The non-classical ion, on the other hand, involves 3 carbons with 2 electrons spread over them. This is called a 3-center 2-electron bond (hypercoordinate bonding) and is a clear marker for a non-classical ion. Notice if you count all of the bonds to any of these 3 carbon atoms (solid and dashed lines) you count 5! Sounds strange, but such "hypercoordinate bonding" is a permitted consequence of the 3-center 2-electron bond.
Mechanism of the Sharpless Epoxidation
Mechanism of the Sharpless Epoxidation
The oxidant for the epoxidation is tert-Butyl hydroperoxide. The reaction is catalyzed by Ti(OiPr)4, which binds the hydroperoxide, the allylic alcohol group, and the asymmetric tartrate ligand via oxygen atoms (putative transition state depicted below).
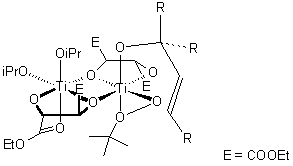
The Sharpless epoxidation is an organic reaction used to steroselectively convert an allylic alcohol to an epoxy alcohol using a titanium isopropoxide catalyst, t-butyl hydroperoxide (TBHP), and a chiral diethyl tartrate (DET). The mechanism begins with the displacement of the isopropoxide ligands on the titanium by DET, TBHP, and finally by the allylic alcohol reagent. This titanium complex is believed to exist as a dimer, but for simplicity is shown as a monomer in the mechanism. Oxidation of the olefin with TBHP then occurs where the chiral DET dictates the face of attack and leads to a steroselective epoxy alcohol.

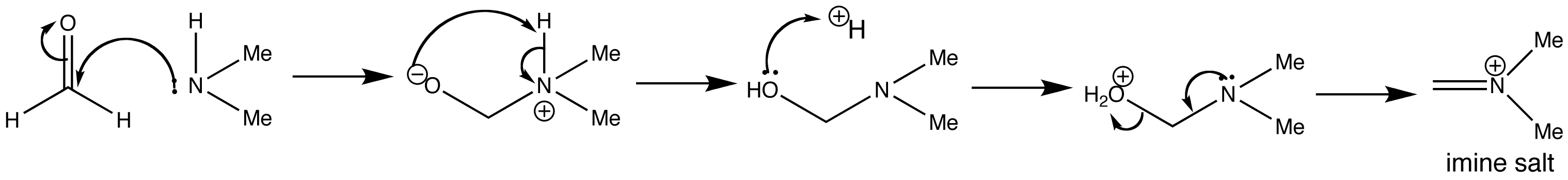
Mannich Reaction
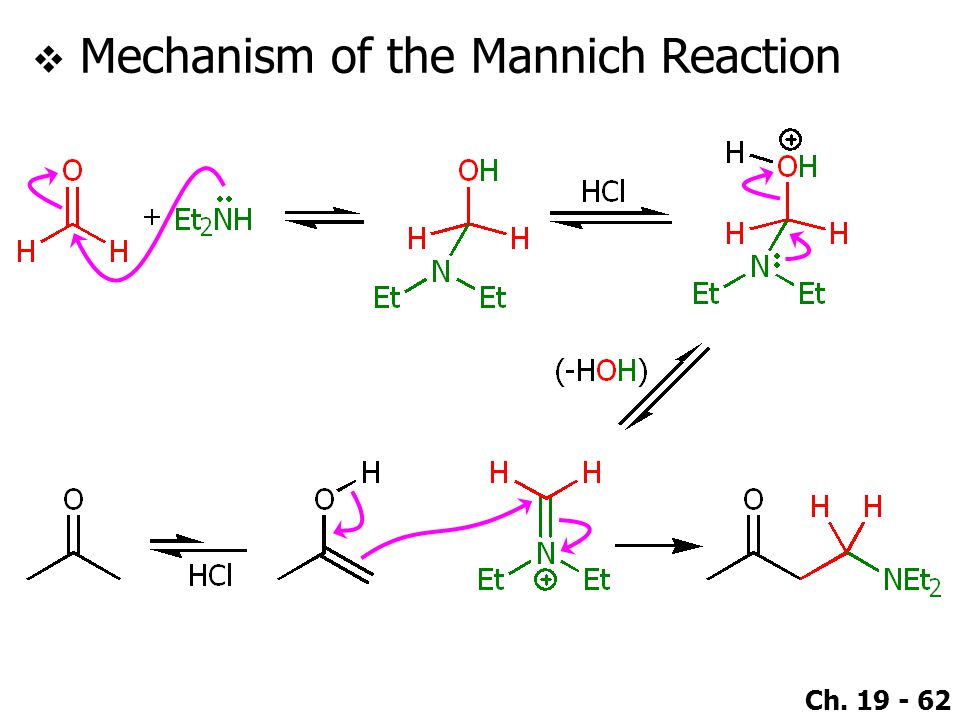
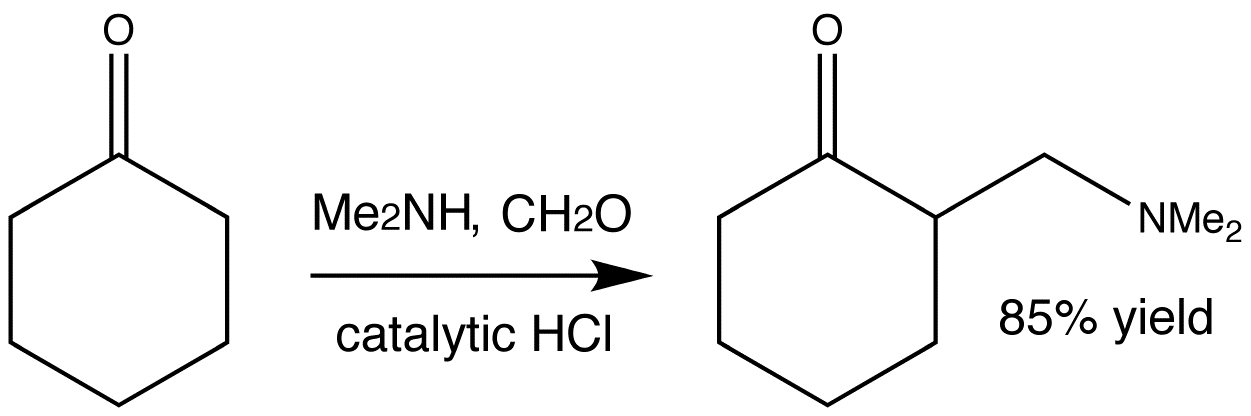
This is a typical example of a Mannich reaction. It involves an enolizable aldehyde or ketone, a secondary amine, formaldehyde as its aqueous solution, and catalytic HCl. The product is an amino-ketone from the addition of one molecule each of formaldehyde and the amine to the ketone.
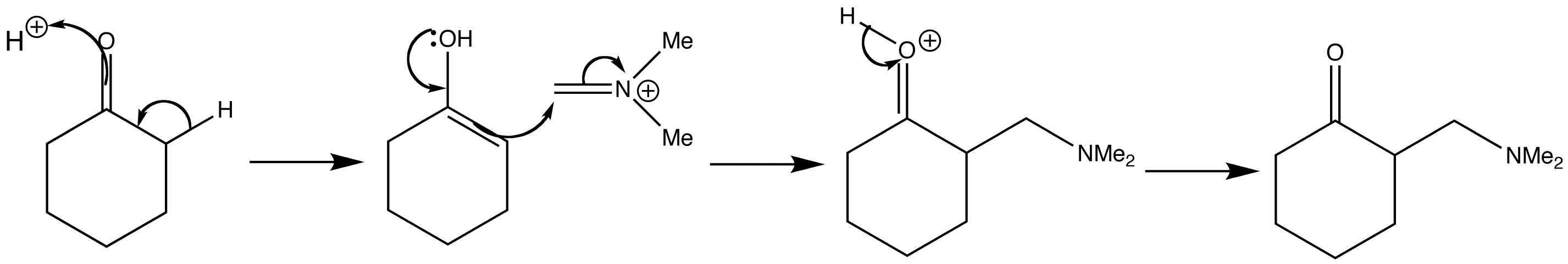
Below are shown the various stages of the Mannich reaction.
Step 1: Imine formation
Step 2: Addition of imine salt to ketone
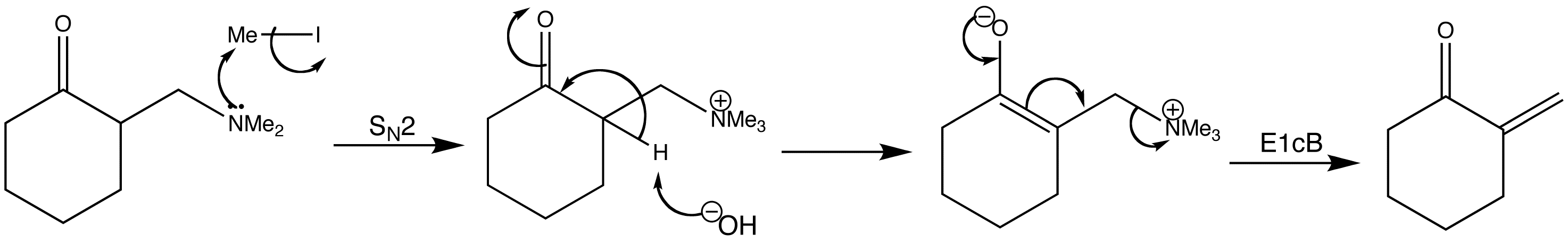
The Mannich products can be converted to enones. Enones such as that shown below, with two hydrogen atoms at the end of the double bond are called exo-methylene compounds. Whilst they are very reactive, they cannot easily be made or stored.
Aldol condensation
Aldol condensation is a reaction that results in the formation of a carbon-carbon bond when an enol (or enolate) molecule reacts with an aldehyde (or ketone). ... And an enolate ion is a compound of the anions, or negatively charged molecules, of an enol.

Mechanism of Condensation Reaction Claisen Condensation Reaction
| Step 1: First, an acid-base reaction. The alkoxide functions as a base and removes the acidic a-hydrogen giving the reactive ester enolate. | 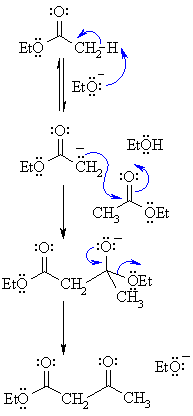 |
| Step 2: The nucleophilic ester enolate attacks the carbonyl C of another ester in a nucleophilic substitution processgiving the tetrahedral intermediate. | |
| Step 3: The intermediate collapses, reforming the C=O, resulting in loss of the leaving group, the alkoxide, leading to the b-ketoester product. | |
The Claisen condensation is an organic reaction used to form a carbon-carbon bond between two ester molecules using an alkoxide base in alcohol to make a β-keto ester. The R group of the ester starting material, the alkoxide base, and the alcohol solvent are chosen to be the same to not end up with a mixture of products. The ester starting material must have an α-hydrogen which is abstracted by the base to form an enolate and alcohol. The enolate then attacks the carbonyl carbon of another ester molecule, an OR group is released to regenerate the base, and the final β-keto ester product is formed. The intramolecular form of the Claisen condensation is the Dieckmann condensation.
| |
Friday, November 3, 2017
Subscribe to:
Posts (Atom)