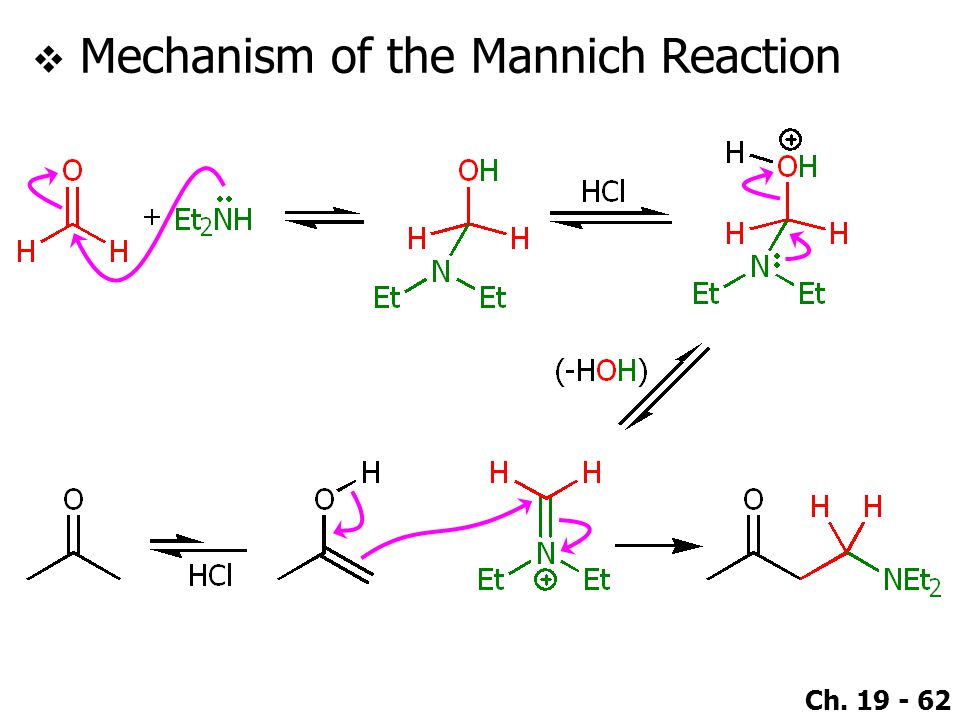
This is a typical example of a Mannich reaction. It involves an enolizable aldehyde or ketone, a secondary amine, formaldehyde as its aqueous solution, and catalytic HCl. The product is an amino-ketone from the addition of one molecule each of formaldehyde and the amine to the ketone.
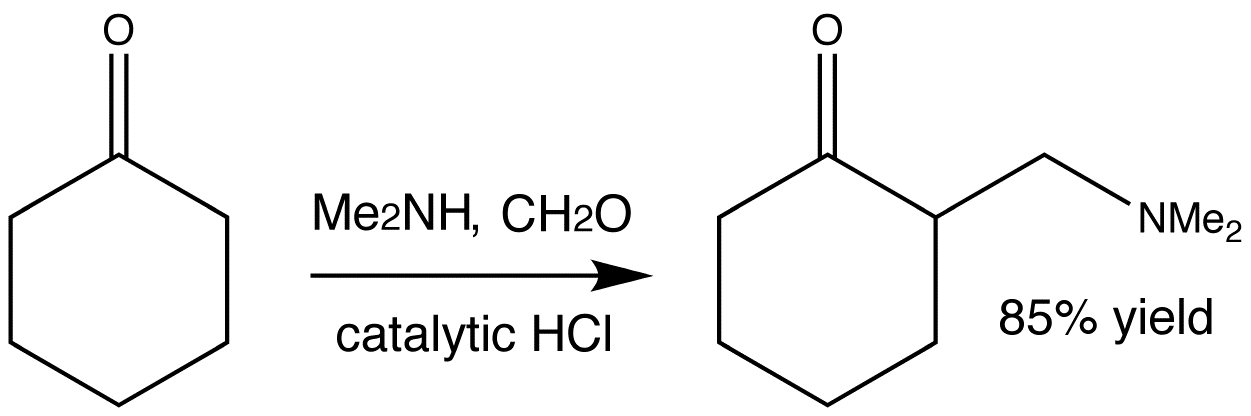
Below are shown the various stages of the Mannich reaction.
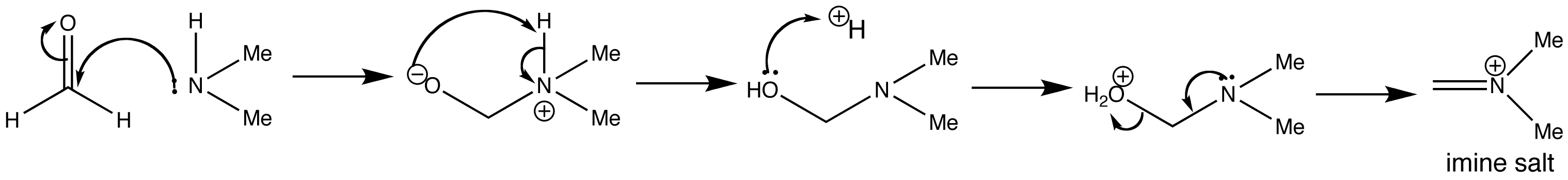
Step 1: Imine formation
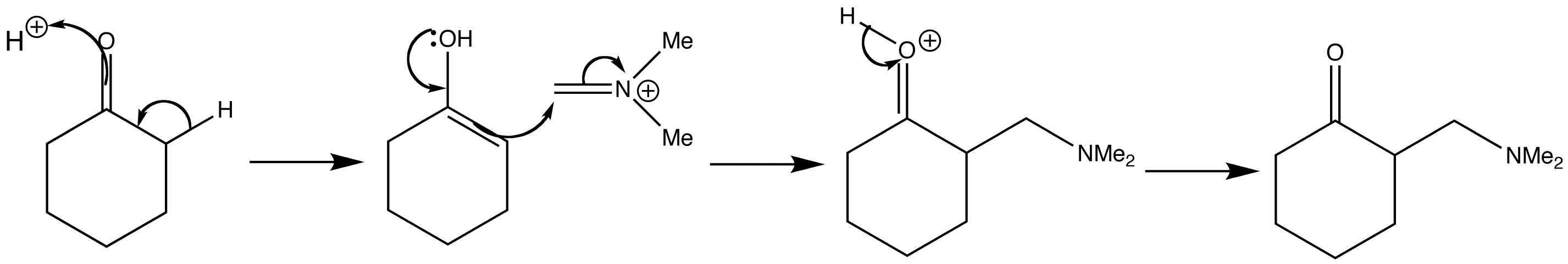
Step 2: Addition of imine salt to ketone
The Mannich products can be converted to enones. Enones such as that shown below, with two hydrogen atoms at the end of the double bond are called exo-methylene compounds. Whilst they are very reactive, they cannot easily be made or stored.
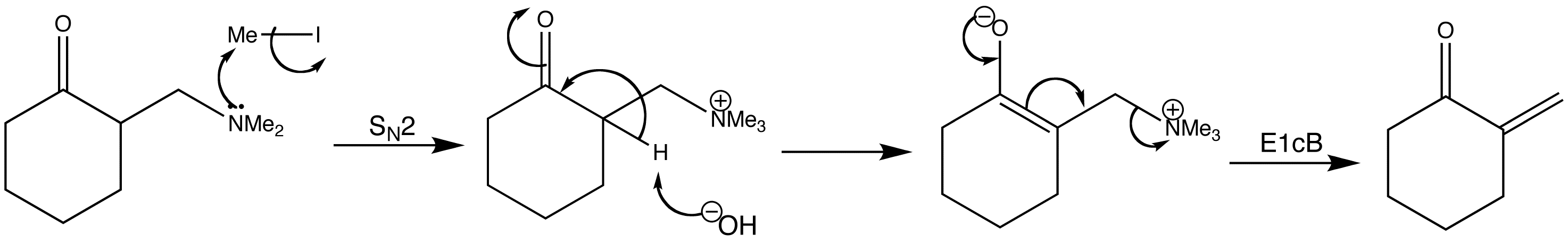

No comments:
Post a Comment