Friday, March 26, 2010
synthetic petrol
Synthetic petrol is manufactured either from coal or from natural gas or from petroleum fraction.Crude oil residues and other residue rich in carbon can be converted into petrol such petrol, too can be called synthetic.
In the process it took 4 tons of coal to produce 1 ton of petrol, pressure up to 700 atm and temperatures of 410° - 460°C being employed. The coal was dried, pulverizing and mixed with heavy oil to form thick slurry. Catalysts were added, and about 70,000 cu. Ft. of hydrogen gas per ton of coal was forced in. the hydrogen was produced from coal and water, the carbon monoxide formed in this process being utilized as fuel gas or converted.
Two important methods for producing synthetic petrol are the Fischer-Tropsch process and the Bergius process. These processes were developed in Germany during World War II, when its petroleum supplies were cut off. Germany produced considerable amounts of fuel from coal by the above processes during that period.
Bergius process
In this process, powdered coal is mixed with heavy oil and heated with hydrogen under high pressure (200-250 atm) at about 748 K in presence of iron oxide as catalyst.
The vapours on condensation give a liquid resembling crude oil. This is called synthetic petroleum, which on fractional distillation gives petrol (gasoline).
Fischer-Tropsch process
In this process, a mixture of water gas and hydrogen under pressure (5-10 atm) is passed over a cobalt catalyst at 450 - 475 K. The water gas required is obtained by passing steam over red-hot coke.
C (red hot) + H2O(g) CO + H2water gas
Sunday, March 14, 2010
Fuels
Fuels
A fuel may be defined as any combustible substance which on burning in presence of oxygen evolved large amount of heat energy.
ORIGIN OF FUEL
Fuel is classified on the basis:-
(I)Occurrence (ii) State of aggregation
On basis of occurrence :-( i) Primary fuels (ii) Secondary fuels
On basic of aggregation: - (i) Solid fuels (ii) Liquid fuels (iii) Gaseous fuel
Chemicals fuels (Occurrence)
↓
↓------------------------------------------------------------------------------↓
Primary fuels (natural) Secondary fuels (Derived)
↓ ↓
↓---------↓---------------↓ ↓----------------↓------------------↓
Solid Liquid Gaseous Solid Liquid Gaseous
Wood Crude Natural Coke Kerosene Coal gas
Coal Oil Gas Charcoal Diesel water gas
LPG
CHARACTERISTICS OF A FUEL
(1) High calorific
(2) Ignition temperature lowest
(3) Moisture content least
(4) Non combustible matter content least
(5) Velocity of combustion moderate
(6) Products of combustion should not be harmful ,On burning should not give out objectionable and harmful gases like Co,So2, H2S, PH3, and CH4.
(7)Low Cost
(8) Smokeless
(9) Control of the process : It can be started or stopped easily.
(10) Easy to transport
(11) Low storage cost
(12) Uniform size.
Monday, February 1, 2010
DEFINE CHOMATOGRAPHY
Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify the mixture or components.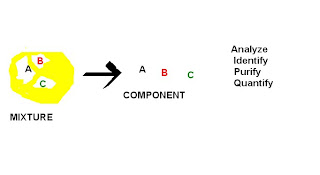
Chromatography is used by scientists to:
• Analyze – examine a mixture, its components, and their relations to one another
• Identify – determine the identity of a mixture or components based on known components
• Purify – separate components in order to isolate one of interest for further study
• Quantify – determine the amount of the a mixture and/or the components present in the sample
Detailed Definition:
Chromatography is a laboratory technique that separates components within a mixture by using the differential affinities of the components for a mobile medium and for a stationary adsorbing medium through which they pass.
Terminology:
• Differential – showing a difference, distinctive
• Affinity – natural attraction or force between things
Definition of Chromatography
Simplified Definition:
Chromatography separates the components of a mixture by their distinctive attraction to the mobile phase and the stationary phase.
Explanation:
• Compound is placed on stationary phase
• Mobile phase passes through the stationary phase
• Mobile phase solubilizes the components
• Mobile phase carries the individual components a certain distance through the stationary phase, depending on their attraction to both of the phases
Wednesday, January 27, 2010
FLASH POINT AND FIRE POINT
FLASH POINT
The flash point of an oil is the lowest temperature at which it gives off vapors that will ignite for a moment when a small flame is brought near it.
FIRE POINT
The fire point of an oil is the lowest temperature at which the vapors of the oil burn continuously
for at least 5 seconds when a flame is brought near it .
MEASURING FLASH POINT
There are two basic types of flash point measurement: open cup and closed cup.
In open cup devices the sample is contained in an open cup which is heated, and at intervals a flame is brought over the surface. The measured flash point will actually vary with the height of the flame above the liquid surface, and at sufficient height the measured flash point temperature will coincide with the fire pont.The best known example is the Cleveland Open Cup (COC).
There are two types of Closed cup testers: non-equilibrium, such as Pensky-Martens where the vapours above the liquid are not in temperature equilibrium with the liquid, and equilibrium, such as Small Scale (commonly known as Setaflash) where the vapors are deemed to be in temperature equilibrium with the liquid. In both these types the cups are sealed with a lid through which the ignition source can be introduced. Closed cup testers normally give lower values for the flash point than Open cup (typically 5-10 °C) and are a better approximation to the temperature at which the vapor pressure reaches the lower flammable limit.
The flash point is an empirical measurement rather than a fundamental physical parameter. The measured value will vary with equipment and test protocol variations, including temperature ramp rate (in automated testers), time allowed for the sample to equilibrate, sample volume and whether the sample is stirred.

Monday, January 11, 2010
Viscosity
Technically, the Viscosity of an oil is a measure of the oils resistance to Shear.
Viscosity is more commonly known as resistance to flow. If a lubricating oil is considered as a series of fluid layers superimposed on each other, the viscosity of the oil is a measure of the resistance to flow between the individual layers. A high viscosity implies a high resistance to flow while a low viscosity indicates a low resistance to flow.
Viscosity varies inversely with temperature.
Viscosity is also affected by pressure; higher pressure causes the Viscosity to increase, and subsequently the Load-Carrying Capacity of the oil also increases. This property enables use of thin oils to lubricate heavy machinery.
Load-Carrying Capacity also increases as operating speed of the lubricated machinery is increased.
Two methods for measuring viscosity are commonly employed: Shear and Time.
(1) Shear
When viscosity is determined by directly measuring shear stress and shear rate, it is expressed in centipoise (cP) and is referred to as the Absolute or Dynamic viscosity. In the oil industry, it is more common to use Kinematic viscosity, which is the absolute viscosity divided by the density of the oil being tested. Kinematic viscosity is expressed in centistokes (cSt). Viscosity in centistokes is conventionally given at two standard temperatures: 40 °C and 100 °C (104 °F and 212 °F ).
(2) Time
Another method used to determine oil viscosity measures the time required for an oil sample to flow through a standard orifice at a standard temperature. Viscosity is then expressed in SUS (Saybolt Universal Seconds). SUS viscosities are also conventionally given at two standard temperatures: 37 °C and 98 °C (100 °F and 210 °). As previously noted, the units of viscosity can be expressed as centipoise (cP), centistokes (cST), or Saybolt Universal Seconds (SUS), depending on the actual test method used to measure the viscosity.
Viscosity Index
The Viscosity Index, commonly designated VI, is an arbitrary numbering scale that indicates the changes in oil viscosity with changes in temperature. Viscosity index can be classified as follows:
Low VI - below 35
Medium VI - 35 to 80
High VI - 80 to 110
Very High VI - 110 to125
Super VI - 125 to 160
Super High VI - above 160 to 200
United Bio Lube's Bio based Oils, Fluids, and Greases all have a Super High Viscosity Index in the range of 150 - 220.
A high Viscosity Index indicates small oil viscosity changes with temperature. A low viscosity index indicates high viscosity changes with temperature. Therefore, a fluid that has a high viscosity index can be expected to undergo very little change in viscosity with temperature extremes and is considered to have a stable viscosity. A fluid with a low viscosity index can be expected to undergo a significant change in viscosity as the temperature fluctuates.
For a given temperature range, say -18 to 370 °C (0 - 100 °F), the viscosity of one oil may change considerably more than another.
An oil with a VI of 95 to 100 would change less than one with a VI of 80. Knowing the viscosity index of an oil is crucial when selecting a lubricant for an application, and is especially critical in extremely hot or cold climates. Failure to use an oil with the proper Viscosity Index when temperature extremes are expected may result in poor lubrication and equipment failure.
Pour Point
The Pour Point is the lowest temperature at which an oil will flow. This property is crucial for oils that must flow at low temperatures. A commonly used rule of thumb when selecting oils is to ensure that the Pour Point is at least 10 °C (20 °F) lower than the lowest anticipated ambient temperature.
Cloud Point
The Cloud Point is the temperature at which dissolved solids in the oil, such as paraffin wax, begin to form and separate from the oil. As the temperature drops, wax crystallizes and becomes visible. Certain oils must be maintained at temperatures above the cloud point to prevent clogging of filters.
Flash Point and Fire Point
The Flash Point is the lowest temperature to which a lubricant must be heated before its vapor, when mixed with air, will ignite but not continue to burn.
The Fire Point is the temperature at which lubricant combustion will be sustained.
The flash and fire points are useful in determining a lubricants Volatility and Fire Resistance. The flash point can be used to determine the transportation and storage temperature requirements for lubricants.
Manufacturers and Toll Blenders can also use the flash point to detect potential product contamination. A lubricant exhibiting a flash point significantly lower than normal will be suspected of contamination with a volatile product. Products with a flash point less than 38 °C (100 °F will usually require special precautions for safe handling. The fire point for a lubricant is usually 8 to 10 percent above the flash point.
The flash point and fire point should not be confused with the Auto-ignition Temperature of a lubricant, which is the temperature at which a lubricant will ignite spontaneously without an external ignition source.
Acid Number or Neutralization Number
The Acid Number or Neutralization Number is a measure of the amount of potassium hydroxide required to neutralize the acid contained in a lubricant. Acids are formed as oils oxidize with age and service. The acid number for an oil sample is indicative of the age of the oil and can be used to determine when the oil must be changed.
Thursday, January 7, 2010
FUNCTION OF LUBRICANTS
In many cases, there is metal-to-metal contact that leads to the generation of friction and heat, which results in wear. The extent of wear in equipment depends upon the degree of the metal-to-metal contact, either due to the equipment design or the nature of the operation. For example, the equipment that is designed to experience minimal metal-to-metal contact, as is the case in most parts of an internal combustion engine, there is little friction and wear. However; the parts that are designed to have intimate metal-to-metal contact, such as gears and bearings, wear due to friction is extensive. With respect to the effect of equipment operation on wear, high-speed, low-load operation leads to lower wear than slow-speed, high-load operation. This is because in the former case there is minimal metal-to-metal contact. A lubricant can be a solid, liquid, or gas, and lubrication is its primary function as we have already discussed in my previous lecture.
The usual objective of the lubrication is to lubricate surfaces to minimize direct metal-to-metal contact and, hence, reduce friction and wear. The term lubricant is also loosely applied to many other fluids that do not specifically perform this function. Examples include power and heat transmission fluids, hydraulic fluids, dielectric fluids, process oils, and the others. Lubricant performs many diverse functions, which help protect and prolong the life of the equipment
FUNCTION OF LUBRICANTS
1. Lubrication (reduce friction and wear)—Lubricant helps reduce friction and wear by introducing a lubricating film between mechanical moving parts, such as gears and bearings. Essentially the presence of a lubricating film minimizes the metal-to-metal contact and reduces the force necessary to move one surface against the other, thereby reducing wear and saving energy.
2. Cooling (heat transfer)—Lubricant acts as a heat sink and dissipates the heat away from the critical moving parts of the equipment, thereby decreasing the possibility of the machine component deformation and wear. The heat is either frictional heat that results from the metal surfaces rubbing against one another, such as in gears, or is conducted and radiated heat, which is due to the close proximity of the parts to a combustion source, such as the combustion chamber in an automobile engine.
3. Cleaning and Suspending—Lubricant facilitates smooth operation of the equipment by removing and suspending potentially harmful products, such as carbon, sludge, and varnish, and the other materials, such as dirt and wear debris. This lubricant function is important in operations that involve high operating temperatures, as in the case of an internal combustion engine or a transmission. This is because in these applications the lubricant gets oxidized to form deposit precursors that can separate on hot surfaces and get converted into deposits.
4. Protection—Lubricant prevents metal damage due to oxidation products, corrosion, and wear. It achieves this by forming a physical film on metal surfaces that is impervious to oxygen, water, and acids, or by forming physical and chemical films by additives, such as rust and corrosion inhibitors, extreme-pressure (EP) additives, and anti-wear agents, that are present in the lubricant.
5. Transfer Power—Lubricant is used as a power transfer medium in some applications, for example, in hydraulic systems. The lubricant performs this function in addition to its normal function of lubrication. Examples of equipment that use hydraulics technology include transmissions, circulating systems, lifts used in automotive service stations, log splitters, fork lifts, dump trucks, and underground continuous mining equipment such as drills, loaders, and miners.
REFERENCE
http://www.astm.org/DIGITAL_LIBRARY/MNL/PAGES/MNL11461M.htm
Klamman, Dieter, Lubricants and Related Products, Verlag Chemie, 1984
G. Corsico, L. Mattei, A. Roselli and C. Gommellini, Poly(internal olefins)- Synthetic Lubricants and high-performance functional fluids,, Marcel Dekker, 1999,Chapter 2, p. 53-62,
R.H. Schlosberg, J.W. Chu, G.A. Knudsen, E.N. Suciu and H.S. Aldrich, High stability esters for synthetic lubricant applications, Lubrication Engineering, February 2001, p. 21-26
Collins (2007), “Implementing Phytoremediation of Petroleum Hydrocarbons, Methods in Biotechnology 23:99-108. Humana Press.
Tuesday, January 5, 2010
THEORIES OF LUBRICANT

When a fluid lubricant is present between two rolling and/or sliding surfaces, a thicker pressurized film can be generated by the movement of the surfaces (velocities). The non-compressible nature of this film separates the surfaces resulting in no metal to metal contact.
- The condition in which surfaces are completely separated by a continuous film of lubricating fluid is commonly referred to as Hydrodynamic or Fluid Film Lubrication .Thickness of the film should be less a1000 angestrom.
- 1.The coefficient of friction is very low.
- 2.The lubricant should have minimum viscosity under working condition .
- 3.Hydrocarbon oils are effective lubricant .
- 4.useful in watches,clocks,sewing machines,working under low load and fair speed of parts.
THIN FILM or BOUNDARY LUBRICATION
Boundary Lubrication (sometimes referred to as thin film lubrication) is a condition in which the lubricant film becomes too thin to provide total separation. This may be due to excessive loading, speeds or a change in the fluid’s characteristics. In such a situation, contact between surface asperities (peaks and valleys) occurs. when a continous film of lubricant cannot persist and direct metal to metal is possible due to certain reasons This happen when a shaft starts moving from rest ,or the speed is low , the load is very high ,vicosity of the oil is too low.
Vegetable and animal oils.,stearic acid ,Oleic acid .
Extreme pressure lubrication :The contact between the metal surfaces increases and more heat is generated due to increased friction .This results in either decompotion or evaporation of liquid lubricant which renders it ineffective .so extreme pressure additives are used along with lubricant .
Additives are chlorine and sulphur in the form of chlorinated waxes and sulphuried fats .
when the moving /sliding surface are under very high pressure and speed ,a high local temperature is attained and under such condition
Defination of Lubricant
Any substance introduced between moving or sliding surface to reduce the friction in order to aviod or reduce the wear and tear is known as lubricant .
The process of reducing friction between two sliding or moving bodies by the introduction of lubricant is known as lubricant is known as lubrication.
